BrainStorm Cell Therapeutics Inc.
In the realm of medical innovation, the quest for effective treatments for devastating diseases is an ongoing challenge. BrainStorm Cell Therapeutics Inc., a biotechnology company specializing in neurodegenerative diseases, has faced a significant setback as the U.S. Food and Drug Administration (FDA) raised concerns over the safety and efficacy of its therapy for amyotrophic lateral sclerosis (ALS), a rare and fatal neurodegenerative disease. The news sent shockwaves through the industry, with the company’s shares plummeting nearly 40.4% to 49 cents in early trading.
Understanding ALS and BrainStorm’s NurOwn:
ALS, often referred to as Lou Gehrig’s Disease, is a devastating condition that affects nerve cells in the brain and spinal cord, leading to progressive muscle weakness and eventually paralysis. Currently, there is no cure for ALS, making the search for effective treatments a top priority in the medical field.
BrainStorm’s therapy, known as NurOwn, had shown promise as a potential treatment for ALS. It is based on stem cell technology and is designed to stimulate the production of healthy nerve cells to replace damaged ones. The therapy had garnered attention and hope as a potential breakthrough in the fight against this debilitating disease.
FDA’s Concerns and Briefing Documents:
However, the recent development involving the FDA has cast a shadow of uncertainty over NurOwn’s future. The FDA’s staff reviewers expressed concerns about the therapy’s safety and efficacy during the regulatory review process. In the FDA’s briefing documents, it was stated that the agency does not believe there is sufficient evidence to support NurOwn’s clinical benefit. Additionally, the documents noted the presence of significant amounts of missing data, which further raised doubts about the therapy’s effectiveness.
These concerns from the FDA are a significant setback for BrainStorm Cell Therapeutics Inc., as they question the fundamental premise of the therapy’s potential to address the unmet medical needs of ALS patients.
Impact on BrainStorm and ALS Patients:
The market’s response to the FDA’s concerns was swift and sharp, with BrainStorm’s shares experiencing a steep decline. This turn of events has not only affected the company’s financial outlook but has also raised questions about the future of NurOwn and the hope it had offered to ALS patients and their families.
ALS is a cruel disease, and any potential treatment that could offer relief or even a cure is eagerly awaited by those affected. The FDA’s concerns underscore the rigorous standards and scrutiny that experimental therapies must undergo to ensure patient safety and efficacy.
Final Outlook
The challenges faced by BrainStorm Cell Therapeutics Inc. in developing a therapy for ALS serve as a stark reminder of the complexities and uncertainties inherent in medical research and innovation. While setbacks are part of the journey, they also emphasize the importance of rigorous scientific evaluation and regulatory scrutiny, especially when it comes to treatments for life-threatening diseases.
The future of NurOwn remains uncertain, and the ALS community, along with the broader medical community, will be closely watching how BrainStorm responds to the FDA’s concerns and whether further research and data can address the regulatory agency’s doubts. Ultimately, the quest for effective ALS treatments continues, driven by the urgency to provide hope and relief to those living with this devastating disease.
Brainstorm Stock Analysis
Analyst Target Price and Rating:
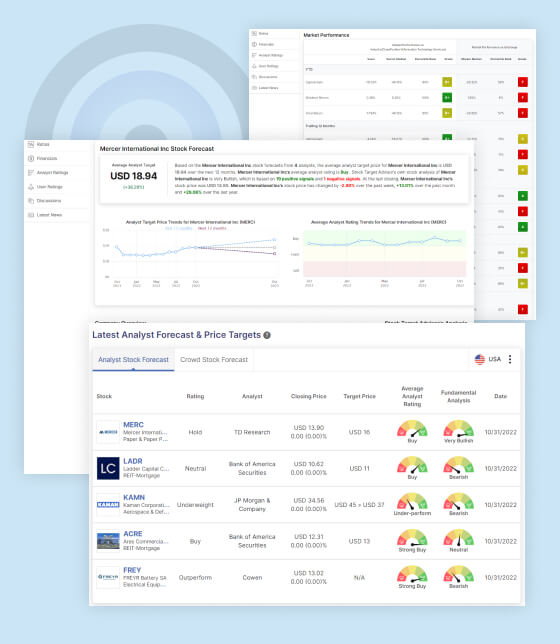
Amidst the uncertainty, one analyst has weighed in on BrainStorm Cell Therapeutics Inc’s future. The average analyst target price projected for the next 12 months stands at USD 10.00, suggesting significant potential for the stock price to appreciate from its current trading price of USD 0.43. This projection reflects an optimistic view from the analyst, who sees potential value in the company’s endeavors.
BrainStorm Cell Therapeutics Inc enjoys an average analyst rating of “Strong Buy.” This rating indicates that the analyst community, on average, holds a positive view of the company’s potential. It suggests that despite recent setbacks, there is confidence in the company’s ability to overcome challenges and deliver on its mission.
Stock Target Advisor’s Analysis: Stock Target Advisor, a stock analysis tool, offers a contrasting perspective. Their analysis leans “Very Bearish,” indicating a pessimistic sentiment regarding BrainStorm Cell Therapeutics Inc’s future. This bearish sentiment is rooted in 0 positive signals and 6 negative signals, which highlight concerns and uncertainties surrounding the company’s current status.
Recent Stock Performance: The recent performance of BrainStorm Cell Therapeutics Inc’s stock is indicative of the challenges it faces:
- Over the Past Week: The company’s stock price experienced a substantial decline of -57.01% in the past week, reflecting the market’s reaction to recent regulatory concerns and uncertainties.
- Over the Past Month: Over the last month, the stock has witnessed an even more significant decline of -74.23%. This steep decline is likely influenced by the intensification of regulatory hurdles and investor sentiment.
- Over the Last Year: In the past year, the stock has seen a staggering drop of -89.82%. This longer-term perspective reflects the company’s struggles in navigating the regulatory landscape and its impact on investor confidence.
Interpreting the Data: The data presented here provides investors with divergent views on BrainStorm Cell Therapeutics Inc’s future. While the average analyst target price and “Strong Buy” rating suggest optimism and potential value, Stock Target Advisor’s “Very Bearish” sentiment and the drastic decline in stock price raise concerns about the company’s ability to address regulatory challenges effectively.