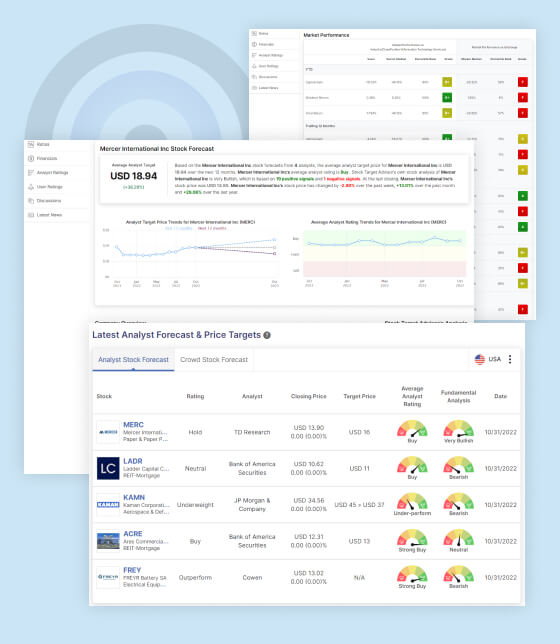
ACADIA Pharmaceuticals Inc Stock Forecast:
According to 17 analysts’ predictions about ACADIA Pharmaceuticals Inc’s stock, the average expected price for the next 12 months is USD 20.54, and the analysts’ overall recommendation is to buy the stock. However, Stock Target Advisor‘s analysis is slightly bearish, based on 4 positive signals and 5 negative signals. ACADIA Pharmaceuticals Inc’s stock closed at USD 19.41 in the last trading session, which represents a decrease of 5.91% over the past week, an increase of 1.20% over the past month, and a decline of 16.26% over the past year.
Analysts Coverage Change:
-
- Jefferies & Company maintains an “Under-Perform” rating for ACADIA (ACAD:NSD) and raises the target price to $15 from $10.
- Mizuho Securities maintains a “Neutral” rating and raises the target price to $20 from $19.
- Guggenheim Securities maintains a “Buy” rating and raises the target price to $25 from $22.
- Cantor Fitzgerald & Co. maintains an “Overweight” rating and raises their target price to $33 from $28.
- H.C. Wainwright maintains a “Buy” rating and raises their target price to $28 from $25.
- Citigroup maintains a “Neutral” rating and raises their target price to $21 from $19.4o.
- Canaccord Genuity maintains a “Buy” rating and raises their target price to $26 from $24.
ACADIA (ACAD:NSD) News:
The recent FDA approval of Acadia Pharmaceuticals Inc’s Daybue (trofinetide) for the treatment of Rett syndrome is a significant milestone in the field of rare genetic neurological and developmental disorders. Rett syndrome is a debilitating disorder that affects primarily females and causes a progressive loss of motor skills and language. The approval of Daybue, the first and only drug approved for treating Rett syndrome, offers hope to the estimated 6,000 to 9,000 patients affected by the disorder in the U.S.
The approval of Daybue was based on the positive results from the Phase 3 LAVENDER study, which demonstrated statistically significant improvement in patients treated with Daybue compared to those treated with a placebo. The drug is expected to be available in the U.S. by the end of April 2023.
Despite the slight drop in Acadia Pharmaceuticals’ stock price following the news, the approval of Daybue is a significant development that offers hope to patients and their families affected by Rett syndrome. The approval of the first drug for this rare disorder is a reminder of the importance of continued research and development in the field of rare diseases.