FDA Approval for Treating Cardiomyopathy
In a significant development for the field of genetic medicine, an outside panel of experts convened by the U.S. Food and Drug Administration (FDA) has given their support for expanding the use of Alnylam Pharmaceuticals Inc.’s gene-silencing drug, patisiran, to treat a specific type of heart disease associated with a rare and potentially organ-damaging disorder. This decision comes after careful consideration of the drug’s efficacy and safety in treating patients with cardiomyopathy linked to transthyretin-mediated amyloidosis.
The Unmet Medical Need: Cardiomyopathy Associated with Amyloidosis
Transthyretin-mediated amyloidosis is a rare condition characterized by the buildup of abnormal proteins called amyloids in various organs, including the heart. When this condition affects the heart, it can lead to a form of cardiomyopathy, a disease of the heart muscle that can result in impaired cardiac function, heart failure, and other serious complications.
Treating such heart conditions effectively has been a long-standing challenge in the medical field. The potential approval of patisiran for this indication brings hope for patients who suffer from these rare and debilitating disorders.
FDA Panel’s Decision and Deliberation
The FDA panel’s vote in favor of patisiran was decisive, with 9 members voting in support of the drug’s expanded use and 3 members opposing it. This majority approval suggests a recognition of patisiran’s potential to address a significant unmet medical need in a patient population that has limited treatment options.
However, the panel’s decision was not without its share of concerns. While the members acknowledged the drug’s benefits, some raised questions about the meaningfulness of these benefits. Dr. Edward Kasper, one of the panelists, aptly captured the sentiment by stating, “There is a light wind for benefit and no wind for risk.”
This cautious stance highlights the panel’s commitment to rigorously assessing the balance between the drug’s efficacy and any potential risks, which is a critical aspect of FDA decision-making.
Patisiran: A Gene-Silencing Breakthrough
Patisiran is part of a groundbreaking class of medications known as RNA interference (RNAi) therapies. It works by silencing or “turning off” specific genes responsible for the production of disease-causing proteins, such as the abnormal transthyretin protein in amyloidosis.
The potential approval of patisiran for the treatment of cardiomyopathy associated with amyloidosis underscores the growing significance of RNAi therapies in modern medicine. These therapies have the potential to address the underlying genetic causes of various diseases, offering hope to patients with rare and challenging conditions.
What Lies Ahead
While the FDA panel’s positive vote is a significant step forward for patisiran and the patients it may benefit, it is important to note that this is just one stage in the regulatory process. The FDA will carefully consider the panel’s recommendations when making its final decision on whether to approve the expanded use of the drug.
If the FDA does grant approval, it will mark a major milestone not only for Alnylam Pharmaceuticals but also for the broader field of genetic medicine. The potential availability of patisiran could significantly improve the lives of individuals living with cardiomyopathy linked to transthyretin-mediated amyloidosis, offering them renewed hope and an opportunity for better health.
As the regulatory process unfolds, the medical community, patients, and investors will be watching closely, recognizing the potential impact of this innovative therapy on the lives of those affected by these rare and challenging conditions.
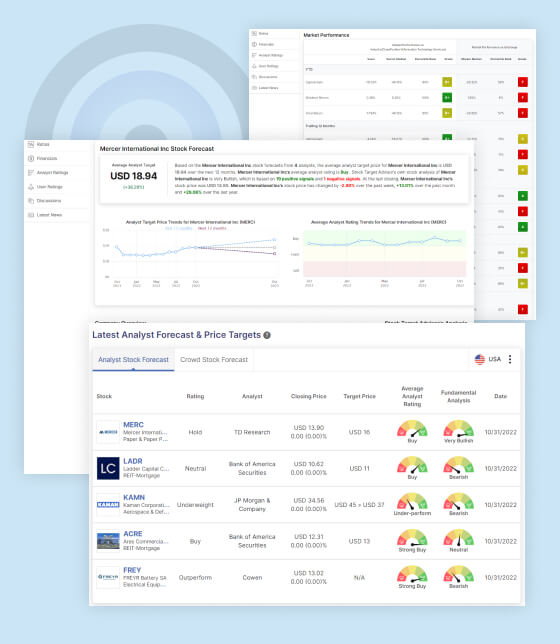
ALNY Stock Analysis & Forecast
Analyst Predictions:
Based on a consensus of 12 analysts, the average target price for Alnylam Pharmaceuticals Inc. is projected to reach USD 249.43 over the next 12 months. This target price represents a significant premium over the current stock price, indicating a favorable outlook for the company’s shares.
The average analyst rating for Alnylam Pharmaceuticals Inc. is classified as “Strong Buy.” This consensus suggests that analysts are not only optimistic about the company’s future prospects but also consider its stock to be undervalued relative to its potential.
Stock Target Advisor’s Analysis:
Stock Target Advisor, an independent analysis platform, provides its own perspective on Alnylam Pharmaceuticals Inc.’s stock. According to their analysis, the outlook for the company is categorized as “Slightly Bullish.” This rating is based on a combination of 5 positive signals and 3 negative signals identified by their proprietary algorithm.
The positive signals may reflect various factors, such as the company’s product pipeline, revenue growth, or positive clinical trial results. Conversely, the negative signals could encompass considerations like market volatility or competitive challenges.
Stock Price Performance:
Understanding the historical performance of a stock is crucial for investors. At the last closing, Alnylam Pharmaceuticals Inc.’s stock price was USD 193.06. This figure represents the most recent valuation of the company’s shares in the stock market.
Over the past week, the stock price experienced a modest decline, down by -4.00%. Short-term fluctuations in stock prices can be influenced by a variety of factors, including market sentiment and macroeconomic events.
However, over the past month, Alnylam Pharmaceuticals Inc.’s stock price showed robust growth, rising by +6.46%. This positive trend could be attributed to a range of factors, including positive news regarding the company’s research and development efforts or broader optimism in the biopharmaceutical sector.
Over the last year, the stock price has seen a decline, down by -7.31%. Long-term stock performance can be influenced by various factors, including market dynamics, regulatory developments, and competitive pressures.
Company Outlook With Approval:
Alnylam Pharmaceuticals Inc. occupies a unique position in the biopharmaceutical industry due to its pioneering work in RNAi therapies. Analysts’ consensus regarding the company’s stock reflects a positive outlook, with a “Strong Buy ” rating and a significantly higher target price compared to the current stock price.